.jpg?sfvrsn=6a306abc_1)
Submit a Proposal
Submitting a proposal for NCDR Research allows researchers to leverage the largest cardiovascular database to explore key clinical questions and improve patient care. Proposals are carefully evaluated to ensure they align with advancing evidence-based cardiovascular research and practice.
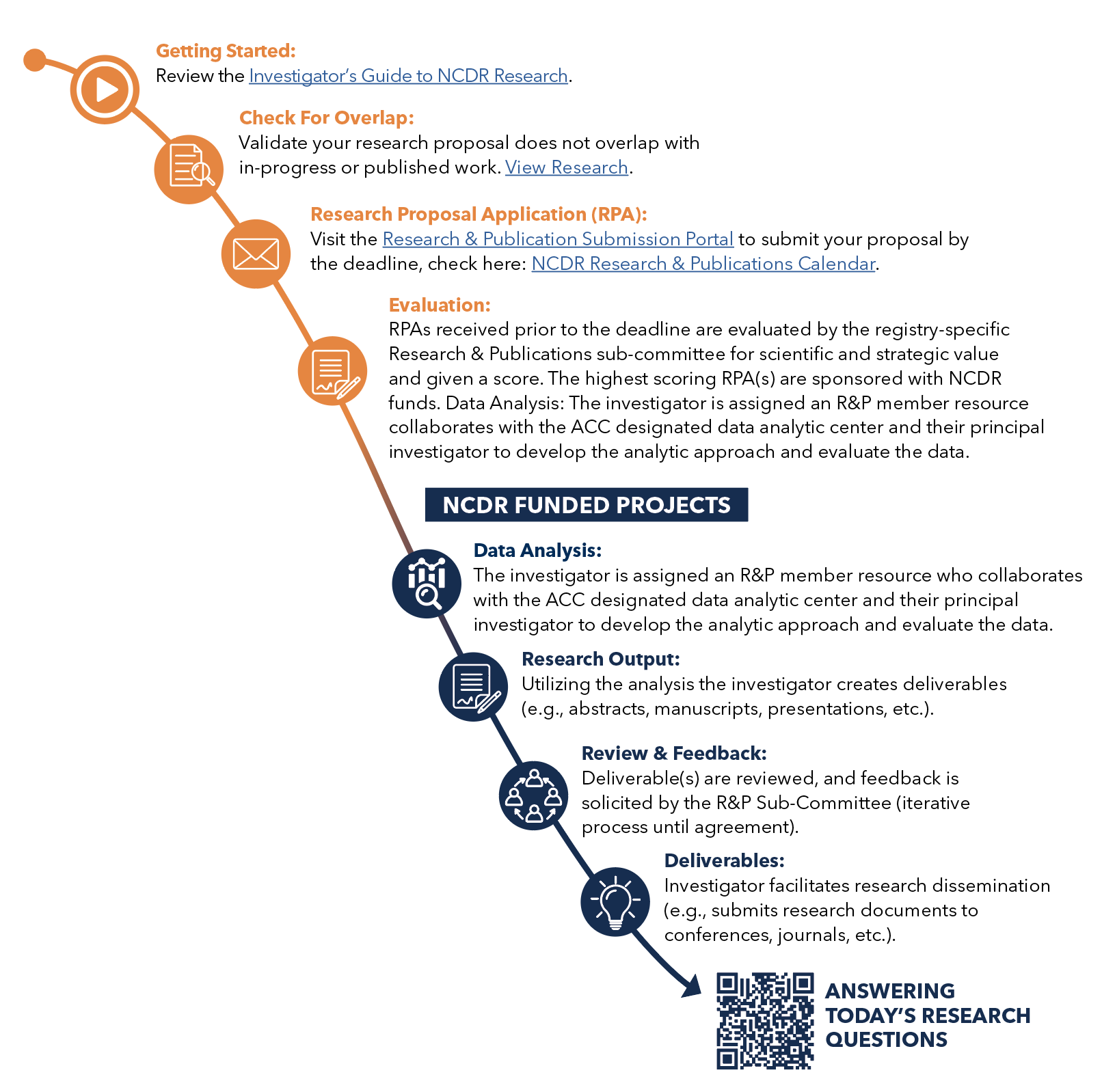
Submitting a Research Proposal for NCDR-Funding Pathway
Attention: New Process For Submitting a Research Proposal
The Research Proposal page is currently being updated. Until the update is complete, please submit your research applications directly to NCDRResearch@acc.org.
How to Submit a Research Proposal:
- Email NCDRResearch@acc.org
- Include the appropriate subject:
- Requesting NCDR Funding
- Applying with External Funding
- Review the NCDR Research Calendar for submission deadlines.
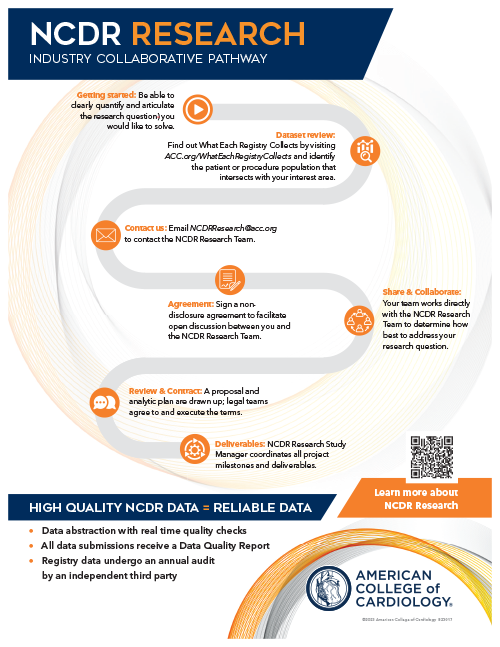
Industry Collaborative Pathway
The NCDR Industry Collaborative Pathway is an ACC initiative designed to foster collaboration between NCDR and industry partners. Through this collaboration, industry partners can access valuable insights and data to inform their research, product development, and clinical trials. The goal is to create a mutually beneficial relationship that advances cardiovascular health and innovation.
To get started, email NCDRResearch@acc.org to contact the NCDR Research Team.
NCDR Mentorship Program
The ACC has established a mentorship program to provide investigators with the opportunity to submit the best research proposal and ensure an equal opportunity for funding. Early career investigators are paired with senior authors to learn from an expert as they navigate the research process.
To request a mentor, email NCDRResearch@acc.org with the subject line "Mentor Request."
Note Regarding JACC Journals
NCDR Research is independent from any scientific journal or meeting. Final decisions regarding choice of target journal are at the discretion of the primary author, and acceptance of manuscripts for publication are at the discretion of the journal's editorial board. Investigators interested in submitting research findings to JACC Journals can visit JACC.org/Submit.
Learn More
For questions email ncdrresearch@acc.org or call 800-257-4737 Monday through Friday between 9 a.m. and 5 p.m. ET.