.jpg?sfvrsn=e8ec6abc_1)
Abstract submissions close March 23. Submit today!
The ACC Quality Summit brings together the cardiovascular care quality improvement community – including clinicians, administrators and quality leaders – to share insights, gain knowledge and drive meaningful improvements in patient care.
Attendees gain actionable strategies and insights through:
- Real-world examples, hands- on sessions and case studies shared by experts.
- Networking and collaboration with peers and quality leaders.
- Leveraging tools and leadership skills to improve team effectiveness and streamline patient care delivery.
Whether you're just starting or leading systemwide change, the Summit offers inspiration, connection, and the resources to help you transform care.
Sign up to be notified when registration opens and use our Justification Letter template to ensure you're with us in Orlando.
Abstract Information
Abstract submissions are officially open! This is your chance to showcase your quality improvement work on a national stage, connect with nearly 1,000 cardiovascular professionals and contribute to advancing patient care.
We invite submissions from all members of the cardiovascular and quality team. Whether you're leveraging NCDR and ACC Accreditation Services data or leading other quality improvement initiatives to enhance patient outcomes, streamline processes or drive innovation in cardiovascular care, we want to hear from you!
Plus, accepted abstracts can be submitted to JACC: Case Reports for publishing consideration. You've done the work, now double your impact. 📓Take a look at last year's special issue spotlighting QI projects to see what your work could be part of.
Need guidance on the submission process? Review the Abstract Submission Process PDF for instructions and key dates.
Submission Deadlines:
📌 Mar. 23 – Consideration for a Live Session Presentation
📌 Mar. 23 – Consideration for an ePoster Presentation
Looking for Inspiration?
- Explore expert-led sessions and best practices in the QII Learning Center or ACC Anywhere
- Review the top 3 eposters from 2025 – First Place | Second Place | Third Place
- Check out this special issue of JACC: Case Reports spotlighting quality improvement projects presented at ACC Quality Summit 2025
- Read this feature article about Quality Summit in the December edition of Cardiology magazine.
📝 Now Recruiting Abstract Reviewers: Want to play a key role in shaping the program? Volunteer as an abstract reviewer and help identify innovative, high-impact QI work from across the community. Apply here by March 24!
CV Team Awards
🏆 CV Team Members — Don't miss your chance to be recognized! Submit for the CVT Quality Summit Travel Award or nominate a colleague for the Distinguished Excellence in Cardiovascular Health Care Quality Award today.
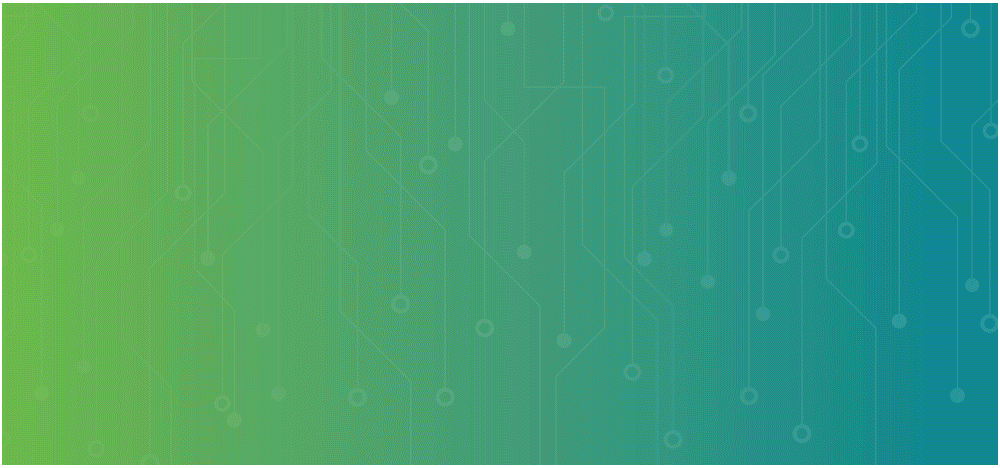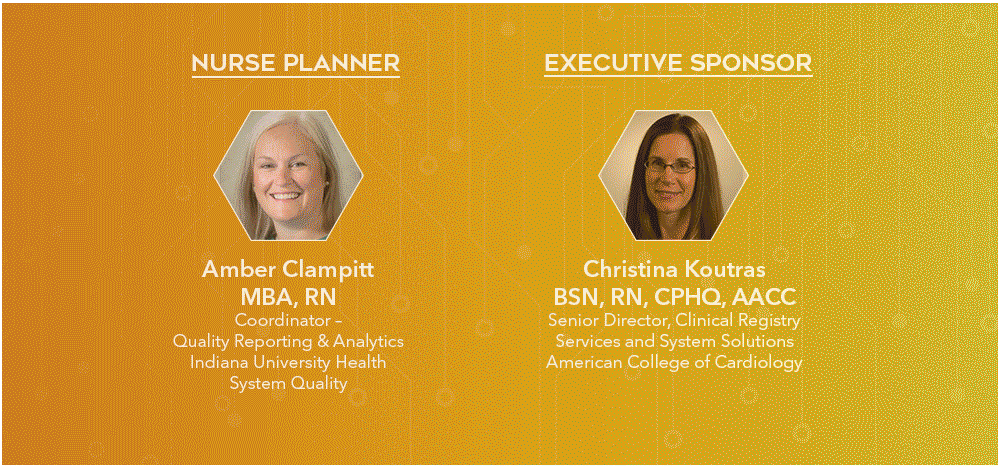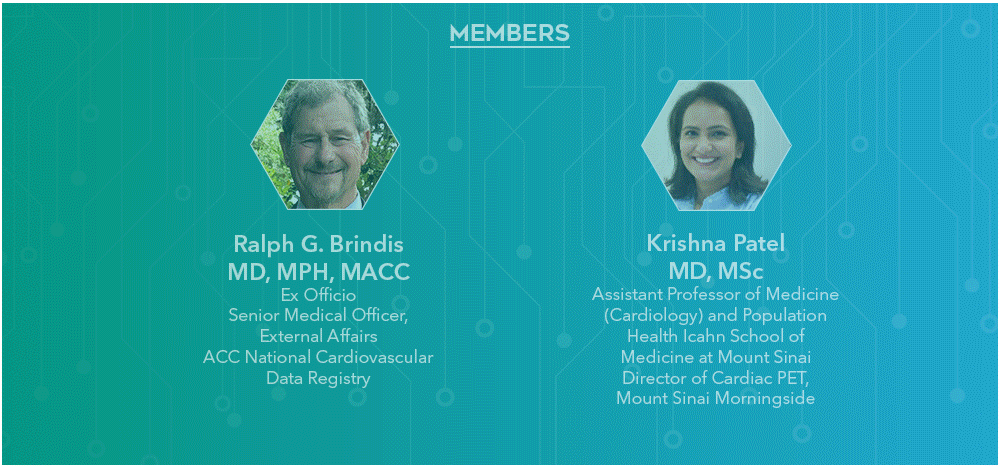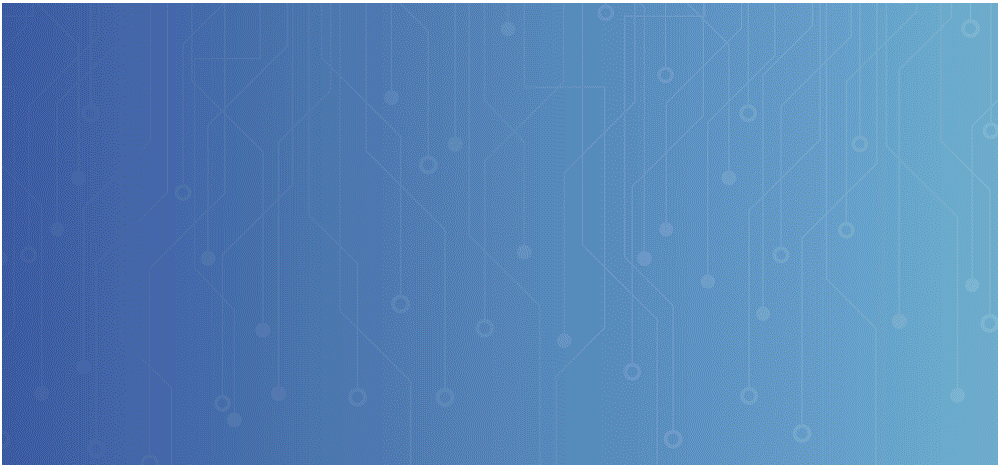
2025 Program Subcommittee
Check out highlights from Quality Summit 2025!
Click here to read the onsite Quality Summit newsletter with highlights from the meeting.






